SCDD1 SUMMARY CORE DATA SET FOR DIABETES
| Change Request |
| Reference: | Change Request 310 |
| Version No: | 1.17 |
| Subject: | DG1114 - Diabetes Reduced Data Set |
| Type of Change: | Revision of NHS data standards |
| Effective Date: | Immediate |
| Reason for Change: | Update NHS Dictionary Version 2 |
The Diabetes Dataset Project was initiated in Autumn 2001 to support the information requirements of the National Service Framework (NSF) for Diabetes and to build upon the recommendations of the Diabetes Information Strategy. A key priority of the Information Strategy is to develop a National Diabetes Dataset with the purpose of allowing the consistent and reliable sharing of information along the diabetes care pathway, and additionally to support clinical audit, performance management and specialist clinical care.
A Diabetes Dataset Advisory Group (DDAG), with wide representation from the colleges, professional bodies, voluntary organisations, patient representatives, and local health care providers, was convened within the remit of the Diabetes Dataset Project. The aim was to build upon the work already undertaken within the diabetes clinical community, so that currently used and existing datasets are developed into a National Diabetes Dataset.
The Objectives of the Diabetes Datasets project are:
1. To develop a Health Management dataset suitable for use by people* with diabetes and their care providers (Diabetic Users Dataset).
2. To develop a Health Management dataset suitable for identification of and use by people* at increased risk of developing diabetes and their care providers (Diabetes At Risk Dataset).
(* understood to include children and their parents but special mention will be made where certain items are inappropriate/ not normally measured/ collected under a certain age)
3. To develop additional specialist & organisational extension datasets to meet the needs of information sharing across a distributed care environment.
Information to support all other health functions, such as public health and prevention programmes, and the use of performance indicators, clinical audit or clinical governance shall be derived from 1), 2) and 3) above.
In line with the patient-centred focus of the Diabetes NSF, the first phase of the Diabetes Dataset Project has resulted in development of the Diabetic Users Dataset (DUD). The DUD defines the minimum standards for the data that is required by a patient, in order to understand and take informed control of their care. The DUD includes all the data items that have been collected as part of the Quality Indicators for Diabetes Services (QUIDS) Project. The data items collected by QUIDS have been developed to produce the Summary Core Dataset for Diabetes.
Summary of changes:| Name: | Norma Southwood |
| Date: | 1 December 2003 |
| Sponsor: | Data Standards Team |
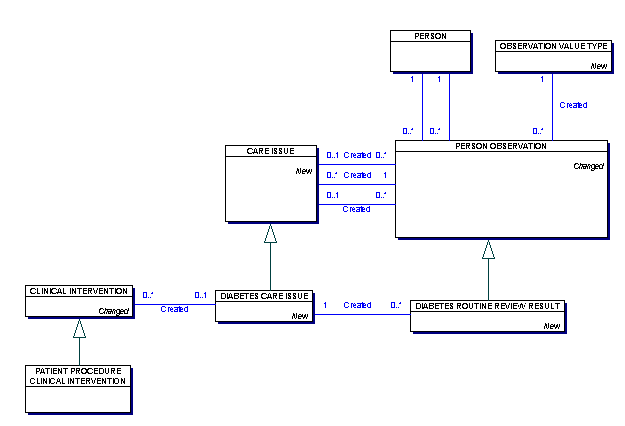
This is a Meta Model Class.
A PERSON OBSERVATION towards which care is directed. It may be a diagnosis, sign, symptom or any other
A single
The CARE ISSUE provides an association between other PERSON OBSERVATIONS and CARE ACTIVITIES that are relevant to that particular issue. For example a blood pressure reading taken during a routine GP visit can be associated with a diabetes
| Context | Alias |
|---|---|
| plural | CARE ISSUES |
Attributes of this Class are:
| K | CARE ISSUE TYPE | |
| K | START DATE | |
| O | END DATE |
Each CARE ISSUE
| must be performed by one and only one PERSON OBSERVATION | |
| may be require one or more PERSON OBSERVATION | |
| may be relevant to one or more PERSON OBSERVATION |
A type of care activity.
Information about medical or surgical actions performed on or planned to be performed on a PATIENT.
References:
The version 1.1 NHS Standard EDIFACT Messages for Pathology Requests and Reports, 2001
The Version 1.0 Trial NHS Standard EDIFACT Messages for GP-Hospital Communications - 17.5.95
| Context | Alias |
|---|---|
| plural | CLINICAL INTERVENTIONS |
Each CLINICAL INTERVENTION
| K | must be associated with one and only one PATIENT |
| may be endocrine therapy for one CANCER CARE SPELL | |
| may be related to one or more CLINICAL INVESTIGATION RESULT ITEM | |
| may be relevant to one DIABETES CARE ISSUE | |
| may be associated with one or more HEALTH CARE PROFESSIONAL INVOLVEMENT | |
| may be classified by one or more INTERVENTION CLASSIFICATION ASSOCIATION | |
| may be related to one SERVICE PROVIDED |
A type of CARE ISSUE.
Diabetes is a chronic and progressive disease that comprises a group of disorders with many different causes, all of which are characterised by a raised blood glucose level. This is the result of a lack of the hormone insulin and/or an inability to respond to insulin. Insulin in the blood, produced by the pancreas, is the hormone which ensures that glucose (sugar) obtained from food can be used by the body. There are two main types of diabetes - Type 1 diabetes and Type 2 diabetes.
| Context | Alias |
|---|---|
| plural | DIABETES CARE ISSUES |
Attributes of this Class are:
| DIABETES TYPE |
Each DIABETES CARE ISSUE
| may be associated with one or more CLINICAL INTERVENTION | |
| may be require one or more DIABETES ROUTINE REVIEW RESULT |
This is a transitory Class that will be replaced as the Generic Core Model and Diabetes Dataset are developed.
A type of PERSON OBSERVATION.
This is a routine review carried out for all young people and adults with diabetes to provide regular surveillance for the long-term complications of diabetes.
| Context | Alias |
|---|---|
| plural | DIABETES ROUTINE REVIEW RESULTS |
Attributes of this Class are:
| DIABETES ROUTINE REVIEW | ||
| DIABETES ROUTINE REVIEW TYPE |
Each DIABETES ROUTINE REVIEW RESULT
| must be review for one and only one DIABETES CARE ISSUE |
This is a transitory Class that will be replaced as the Generic Core Model is developed.
Classifies the type of value entered in the OBSERVATION VALUE.
| Context | Alias |
|---|---|
| plural | OBSERVATION VALUE TYPES |
Attributes of this Class are:
| K | VALUE TYPE |
Each OBSERVATION VALUE TYPE
| may be classifier for one or more PERSON OBSERVATION |
Attributes of this Class are:
| K | PERSON OBSERVATION NUMBER | |
| O | ALBUMINURIA STAGE | |
| Required for person OBSERVATION TYPE '07 - Urinary Albumin Level' | ||
| O | END DATE | |
| O | END TIME | |
| OBSERVATION DATE | ||
| OBSERVATION TYPE | ||
| OBSERVATION VALUE | ||
| START TIME |
Each PERSON OBSERVATION
| K | must be an observation of one and only one PERSON |
| must be observed by one and only one HEALTH CARE PROFESSIONAL | |
| or must be observed by one and only one PERSON | |
| must be classified by one and only one OBSERVATION VALUE TYPE | |
| may be required by one CARE ISSUE | |
| may be of relevance to one CARE ISSUE | |
| may be act as one or more CARE ISSUE | |
| may be the observation in one or more PERSON OBSERVATION WITHIN CARE SPELL | |
| may be related to one SERVICE PROVIDED |
The level of albuminuria in relation to risk of diabetic renal disease and/or cardiovascular disease. Identifies renal and cardiovascular risk for a person with diabetes.
National Codes:
| 01 | Normalalbuminuria |
| 02 | Microalbuminuria |
| 03 | Macroalbuminuria |
| Context | Alias |
|---|---|
| plural | ALBUMINURIA STAGES |
Classifies the type of CARE ISSUE identified and which may be the focus of a CARE SPELL. This is derived from the PERSON OBSERVATION that acts as the Care Issue for example Diabetes, High Blood Pressure etc.
| Context | Alias |
|---|---|
| plural | CARE ISSUE TYPES |
A record about the regular DIABETES ROUTINE REVIEW RESULT for a person with diabetes.
National Codes:
| 01 | Carried out |
| 02 | Not done |
| 03 | Not necessary |
| Context | Alias |
|---|---|
| plural | DIABETES ROUTINE REVIEWS |
Identifies the type of regular DIABETES ROUTINE REVIEW RESULT for a person with diabetes.
National Codes:
| 01 | Eye Examination |
| 02 | Foot Examination |
| Context | Alias |
|---|---|
| plural | DIABETES ROUTINE REVIEW TYPES |
Classifies the type of diabetes experienced by the PATIENT.
National Codes:
| 01 | Type 1 |
| 02 | Type 2 |
| 06 | MODY |
| 08 | Other Specified |
| 99 | Not specified |
'99 - Not Specified' should only be used in the short term and refined to one of the alternative options as soon as possible.
| Context | Alias |
|---|---|
| plural | DIABETES TYPES |
The date on which a PERSON OBSERVATION was observed.
| Context | Alias |
|---|---|
| plural | OBSERVATION DATES |
Identifies the type of PERSON OBSERVATION observed during a CARE ACTIVITY.
National Codes:
| 01 | Blood Pressure |
| 02 | Body Mass Index |
| 03 | Patient Diagnosis |
| 04 | Serum Cholesterol Level |
| 05 | Serum Creatinine Level |
| 06 | HbA1c Level |
| 07 | Urinary Albumin Level |
| 08 | Diabetes Routine Review |
| 09 | Cardiac Arrest |
| 10 | Acute Myocardial Infarction History Item |
| Context | Alias |
|---|---|
| plural | OBSERVATION TYPES |
The recorded value for the PERSON OBSERVATION made.
| Context | Alias |
|---|---|
| plural | OBSERVATION VALUES |
The type of value recorded for a PERSON OBSERVATION.
National Codes:
| 01 | mmol/L |
| 02 | µmol/L |
| 03 | µg/L |
| 04 | µg/mmol |
| 05 | µg/ml/hr |
| 06 | µg/min |
| 07 | µg/24hr |
| 08 | Number |
| 09 | Percentage |
| Context | Alias |
|---|---|
| plural | VALUE TYPES |
| Format/length: | n2 |
| HES item: | |
| National Codes: | Press Definition button for the National Codes |
| Default Codes: |
Notes:
| Context | Alias |
|---|---|
| plural | ALBUMINURIA STAGES |
| Format/length: | n8 - ccyymmdd |
| HES item: | |
| National Codes: | |
| Default Codes: |
Notes:
This records the date on which a PERSON died, as recorded on the Death Certificate and corresponds to DEATH DATE.
| Context | Alias |
|---|---|
| plural | DEATH DATES |
| Format/length: | n2 |
| HES item: | |
| National Codes: | Press Definition button for the National Codes |
| Default Codes: |
Notes:
A DIABETES ROUTINE REVIEW of the eyes within an approved diabetes eye screening programme.
| Context | Alias |
|---|---|
| plural | DIABETES ROUTINE REVIEWS (EYE) |
| Format/length: | n2 |
| HES item: | |
| National Codes: | Press Definition button for the National Codes |
| Default Codes: |
Notes:
A DIABETES ROUTINE REVIEW of the foot carried out by appropriately trained personnel using an approved foot screening procedure.
| Context | Alias |
|---|---|
| plural | DIABETES ROUTINE REVIEWS (FOOT) |
| Format/length: | n2 |
| HES item: | |
| National Codes: | Press Definition button for the National Codes |
| Default Codes: |
| Context | Alias |
|---|---|
| plural | DIABETES TYPES |
| Format/length: | n8 - ccyymmdd |
| HES item: | |
| National Codes: | |
| Default Codes: |
Notes:
Diagnosis Date (Diabetes) is the same as OBSERVATION DATE for the sub type PATIENT DIAGNOSIS where PRIMARY DIAGNOSIS is 'diabetes'.
| Context | Alias |
|---|---|
| plural | DIAGNOSIS DATES (DIABETES) |
| Format/length: | annnaa for ICD - 10 (see 10th Revision) |
| HES item: | |
| National Codes: | |
| Default Codes: |
Notes:
Records the DIAGNOSTIC CODING identified in the following relevant conditions and complications associated with the diabetic condition: The list shows those conditions and complications currently extracted by QUIDS using data linkage to Hospital Episode Statistics (HES).
ICD-10 Codes for relevant conditions and complications
| HYPERDKA ( Hyperglycaemic emergencies) | |
| E10.1 | Insulin-dependent diabetes mellitus with ketoacidosis |
| E11.1 | Non-insulin-dependent diabetes mellitus with ketoacidosis |
| E13.1 | Other specified diabetes mellitus with ketoacidosis |
| E14.1 | Unspecified diabetes mellitus with ketoacidosis |
| E10.0 | Insulin-dependent diabetes mellitus with ketoacidosis and coma |
| E11.0 | Non-insulin-dependent diabetes mellitus with ketoacidosis and coma |
| E13.0 | Other specified diabetes mellitus with coma |
| E14.0 | Unspecified diabetes mellitus with coma |
| ANGINA | |
| I20.0 | Unstable angina |
| I20.1 | Angina pectoris with documented spasm |
| I20.8 | Other forms of angina pectoris |
| I20.9 | Angina pectoris |
| MI (Myocardial Infarction) | |
| I21.0 | Acute transmural myocardial infarction of anterior wall |
| I21.1 | Acute transmural myocardial infarction of inferior wall |
| I21.2 | Acute transmural myocardial infarction of other sites |
| I21.3 | Acute transmural myocardial infarction of unspecified site |
| I21.4 | Acute subendocardial myocardial infarction |
| I21.9 | Acute myocardial infarction |
| I22.0 | Subsequent myocardial infarction of anterior wall |
| I22.1 | Subsequent myocardial infarction of inferior wall |
| I22.8 | Subsequent myocardial infarction of other sites |
| I22.9 | Subsequent myocardial infarction of unspecified site |
| CF (Cardiac Failure) | |
| I50.0 | Congestive heart failure. |
| I50.1 | Left ventricular failure. |
| I50.9 | Heart failure unspecified. |
| CVA (Stroke / Cerebro-Vascular Accident) | |
| I61.0 | Intracerebral haemorrhage in hemisphere, subcortical |
| I61.1 | Intracerebral haemorrhage in hemisphere, cortical |
| I61.2 | Intracerebral haemorrhage in hemisphere, unspecified |
| I61.3 | Intracerebral haemorrhage in brain stem |
| I61.4 | Intracerebral haemorrhage in cerebellum |
| I61.5 | Intracerebral haemorrhage, intraventricular |
| I61.6 | Intracerebral haemorrhage, multiple localised |
| I61.8 | Other intracerebral haemorrhage |
| I61.9 | Intracerebral haemorrhage, unspecified |
| I63.0 | Cerebral infarct due to thrombosis of precerebral arteries |
| I63.1 | Cerebral infarction due to embolism of precerebral arteries |
| I63.2 | Cerebral infarct due to unspecified occlusion or stenos of precerebral arteries |
| I63.3 | Cerebral infarction due to thrombosis of cerebral arteries |
| I63.4 | Cerebral infarction due to embolism of cerebral arteries |
| I63.5 | Cerebral infarction due to unspecified occlusion or stenosis of cerebral arteries |
| I63.6 | Cerebral infarction due to cerebral venous thrombosis |
| I63.8 | Other cerebral infarction |
| I63.9 | Cerebral infarction, unspecified |
| I64.X | Stroke, unspecified |
| RRT (End stage renal failure requiring renal replacement therapy) | |
| N18.0 | End-stage renal disease |
| Z99.2 | Dependence on renal dialysis |
| Z49.0 | Preparatory care for dialysis |
| Z49.1 | Extracorporeal dialysis |
| Z49.2 | Other dialysis |
| Context | Alias |
|---|---|
| plural | DIAGNOSTIC CODING (DIABETES RELEVANT ICD-10) |
| Format/length: | an7 for The Read Codes |
| HES item: | |
| National Codes: | |
| Default Codes: |
Notes:
Records the DIAGNOSTIC CODING identified in the following relevant conditions and complication associated with the diabetic condition:
Read Codes (diagnosis)
| DKA ( Hyperglycaemic emergencies) 4-byte | |
| C2... | Diabetes mellitus Ketoacidosis - diabetic (synonym) |
| C24.. | Diabetes mellitus + ketoacidosis - no coma |
| C25.. | Diabetes with coma |
| Version 2 | |
| C101. | Diabetes mellitus with ketoacidosis |
| C1010 | Diabetes mellitus, juvenile type, with ketoacidosis |
| C1011 | Diabetes mellitus, adult onset, with ketoacidosis |
| C101y | Other specified diabetes mellitus with ketoacidosis |
| C101z | Diabetes mellitus NOS with ketoacidosis |
| ANGINA 4-byte | |
| G44. | Angina pectoris |
| G440. | Unstable angina |
| G444. | Stable angina |
| Version 2 | |
| G3111 | Unstable angina |
| G33.. | Angina pectoris |
| G33z. | Angina pectoris NOS |
| G33z7 | Stable angina |
| MI (Myocardial Infarction 4-byte | |
| G6A. | Heart failure (preferred term), Cardiac failure (synonym) |
| G6A1. | Congestive cardiac failure |
| Version 2 | |
| G58.. | Heart failure (preferred term) Cardiac failure (synonym) |
| G580. | Congestive cardiac failure |
| CVA (Stroke/Cerebro-Vascular Accident) 4-byte | |
| G7.. | Cerebrovascular disease |
| G712. | Intracerebral haemorrhage |
| G73.. | Cerebral arterial occlusion |
| G75.. | Stroke/CVA undefined |
| Version 2 | |
| G6... | Cerebrovascular disease |
| G61.. | Intracerebral haemorrhage |
| G64.. | Cerebral arterial occlusion |
| G66.. | Stroke/CVA unspecified |
| RRT (End stage renal failure requiring renal replacement therapy) 4-byte | |
| J16. | Chronic renal failure (perferred term) End stage renal failure (synonym) |
| Version 2 | |
| K050. | End stage renal failure |
| Context | Alias |
|---|---|
| plural | DIAGNOSTIC CODING (DIABETES RELEVANT READ CODES) |
| Format/length: | n3 - nnn |
| HES item: | |
| National Codes: | |
| Default Codes: |
Notes:
The recorded sitting blood pressure after 2 minutes rest, at 5th phase (mm Hg).
| Context | Alias |
|---|---|
| plural | DIASTOLIC BLOOD PRESSURE |
| Format/length: | n8 - ccyymmdd |
| HES item: | |
| National Codes: | |
| Default Codes: |
Notes:
The OBSERVATION DATE when the OBSERVATION TYPE 'BLOOD PRESSURE' was taken.
| Context | Alias |
|---|---|
| plural | OBSERVATION DATES (BLOOD PRESSURE) |
| Format/length: | n8 - ccyymmdd |
| HES item: | |
| National Codes: | |
| Default Codes: |
Notes:
The OBSERVATION DATE when the OBSERVATION TYPE ' Body Mass Index' was calculated.
| Context | Alias |
|---|---|
| plural | OBSERVATION DATES (BMI) |
| Format/length: | n8 - ccyymmdd |
| HES item: | |
| National Codes: | |
| Default Codes: |
Notes:
The OBSERVATION DATE when the DIAGNOSTIC CODING (DIABETES RELEVANT ICD-10) or DIAGNOSTIC CODING (DIABETES RELEVANT READ CODES) was first recognised or the date on which a diabetic complication occurred.
| Context | Alias |
|---|---|
| plural | OBSERVATION DATES (DIABETES RELEVANT DIAGNOSIS) |
| Format/length: | n8 - ccyymmdd |
| HES item: | |
| National Codes: | |
| Default Codes: |
Notes:
The OBSERVATION DATE when the DIABETES ROUTINE REVIEW (EYE) took place.
| Context | Alias |
|---|---|
| plural | OBSERVATION DATES (EYE EXAMINATION) |
| Format/length: | n8 - ccyymmdd |
| HES item: | |
| National Codes: | |
| Default Codes: |
Notes:
The OBSERVATION DATE when the DIABETES ROUTINE REVIEW (FOOT) took place.
| Context | Alias |
|---|---|
| plural | OBSERVATION DATES (FOOT EXAMINATION) |
| Format/length: | n8 - ccyymmdd |
| HES item: | |
| National Codes: | |
| Default Codes: |
Notes:
The OBSERVATION DATE for the OBSERVATION TYPE 'HbA1c level'.
| Context | Alias |
|---|---|
| plural | OBSERVATION DATES (HbA1c LEVEL) |
| Format/length: | n8 - ccyymmdd |
| HES item: | |
| National Codes: | |
| Default Codes: |
Notes:
The OBSERVATION DATE for the OBSERVATION TYPE 'serum cholesterol level'.
| Context | Alias |
|---|---|
| plural | OBSERVATION DATES (SERUM CHOLESTEROL LEVEL) |
| Format/length: | n8 - ccyymmdd |
| HES item: | |
| National Codes: | |
| Default Codes: |
Notes:
The OBSERVATION DATE for the OBSERVATION TYPE 'serum creatinine level'.
| Context | Alias |
|---|---|
| plural | OBSERVATION DATES (SERUM CREATININE LEVEL) |
| Format/length: | n8 - ccyymmdd |
| HES item: | |
| National Codes: | |
| Default Codes: |
Notes:
The START DATE when the SMOKING STATUS was recorded.
| Context | Alias |
|---|---|
| plural | OBSERVATION DATES (SMOKING STATUS) |
| Format/length: | n8 - ccyymmdd |
| HES item: | |
| National Codes: | |
| Default Codes: |
Notes:
The OBSERVATION DATE for the OBSERVATION TYPE 'urinary albumin level' .
| Context | Alias |
|---|---|
| plural | OBSERVATION DATES (URINARY ALBUMIN LEVEL) |
| Format/length: | n3 nn.n |
| HES item: | |
| National Codes: | |
| Default Codes: |
Notes:
This records the Body Mass Index of the PERSON and corresponds to OBSERVATION VALUE where the OBSERVATION TYPE = 'Body Mass Index' and the VALUE TYPE = 'Number'.
This value is derived from WEIGHT in kilograms devided by HEIGHT in metres squared (kg/m²).
| Context | Alias |
|---|---|
| plural | BMI |
| Format/length: | n3 nn.n |
| HES item: | |
| National Codes: | |
| Default Codes: |
Notes:
The recorded glycated haemoglobin and corresponds to OBSERVATION VALUE where the OBSERVATION TYPE = "HbA1c Level" and the VALUE TYPE = "number".
| Context | Alias |
|---|---|
| plural | PERSON OBSERVATIONS (HbA1c LEVEL) |
| Format/length: | n3 nn.n |
| HES item: | |
| National Codes: | |
| Default Codes: |
Notes:
The recorded cholesterol level (mmol/L) and corresponds to OBSERVATION VALUE where the OBSERVATION TYPE = ''Serum Cholesterol Level;" and the VALUE TYPE = "mmol/L".
| Context | Alias |
|---|---|
| plural | PERSON OBSERVATIONS (SERUM CHOLESTEROL LEVEL) |
| Format/length: | n4 - nnnn |
| HES item: | |
| National Codes: | |
| Default Codes: |
Notes:
The recorded creatinine (µmol/L): serum creatinine using laboratory assay and corresponds to OBSERVATION VALUE where the OBSERVATION TYPE = "Serum Creatinine Level" and the VALUE TYPE = "µmol/L".
| Context | Alias |
|---|---|
| plural | PERSON OBSERVATIONS (SERUM CREATININE LEVEL) |
| Format/length: | n3 - nnn |
| HES item: | |
| National Codes: | |
| Default Codes: |
Notes:
The recorded result of the urinary albumin level must be accompanied by a recorded URINARY ALBUMIN LEVEL TESTING METHOD.
Derive from the OBSERVATION VALUE recorded for the OBSERVATION TYPE 'Urinary Albumin level'.
| Context | Alias |
|---|---|
| plural | PERSON OBSERVATIONS (URINARY ALBUMIN LEVEL) |
| Format/length: | annn for OPCS-4 |
| HES item: | |
| National Codes: | |
| Default Codes: |
Notes:
Records the PROCEDURE CODING identified in the following relevant procedures associated with the diabetic condition.
OPCS-4 Codes
| LASER (Ocular retinal photocoagulation) | |
| C82.1 | Cauterisation of lesion of retina |
| Y08.4 | Laser destruction (secondary procedure) |
| MINOR AMPUTATION (amputation toe or below ankle) | |
| X11.1 | Amputation of great toe |
| X11.2 | Amputation of phalanax of toe |
| X11.8 | Amputation of toe, other specified |
| X11.9 | Amputation of toe, unspecified |
| X10.1 | Amputation of foot through ankle |
| X10.8 | Amputation of foot, other specified |
| X10.9 | Amputation of foot, unspecified |
| MAJOR AMPUTATION (amputation leg, above or below knee) | |
| X09.3 | Amputation of leg above knee |
| X09.4 | Amputation of leg through knee |
| X09.5 | Amputation of leg below knee |
| RRT (End stage renal failure requiring renal replacement therapy) | |
| X40.1 | Compensation for renal failure, renal dialysis |
| X40.2 | Compensation for renal failure, peritineal dialysis |
| X40.3 | Compensation for renal failure, haemodialysis |
| X40.8 | Compensation for renal failure, other specified |
| X40.9 | Compensation for renal failure, unspecified |
| M01.1 | Transplantation of kidney autotransplantation of kidney |
| M01.2 | Transplantation of kidney allotransplantation of kidney |
| M01.3 | Transplantation of kidney allotransplantation of kidney |
| M01.8 | Transplantation of kidney other specified |
| M01.9 | Transplantation of kidney unspecified |
| Context | Alias |
|---|---|
| plural | PROCEDURE CODING (DIABETES RELEVANT OPCS-4) |
| Format/length: | an7 for the Read Codes |
| HES item: | |
| National Codes: | |
| Default Codes: |
Notes:
Records the PROCEDURE CODING identified in the following relevant procedures associated with the diabetic condition.
Read Codes
| LASER (Ocular retinal photocoagulation) 4-byte | |
| 8633 | Retinal photocoag. therapy |
| Version 2 | |
| No equivalent term | |
| AMPUTATION 4-byte | |
| 7EU.. | Amputation - lower limb |
| 7EU1. | Amputation of toes |
| 7EU2. | Amputation foot: tarsal-metatar |
| 7EU3. | Amputation foot: mid-tarsal |
| 7EU5. | Supramalleolar ankle amptat. |
| 7EU6. | Below knee amputaion |
| 7EU7. | Above knee amputation |
| Version 2 | |
| 7L06. | Amputation of leg |
| 7L07. | Amputation of foot |
| 7L08. | Amputation of toe |
| RRT (End stage renal failure requiring renal replacement therapy) 4-byte | |
| 8874 | Haemodialysis (preferred term) Dialysis - renal (synonym) |
| 7A4 | Kidney Transplant |
| Version 2 | |
| 7L1A. | Compensation for renal failure |
| 7B00. | Kidney Transplant |
| Context | Alias |
|---|---|
| plural | PROCEDURE CODING (DIABETES RELEVANT READ) |
| Format/length: | n3 - nnn |
| HES item: | |
| National Codes: | |
| Default Codes: |
Notes:
The recorded sitting blood pressure after 2 minutes rest, at 1st phase (mm Hg).
| Context | Alias |
|---|---|
| plural | SYSTOLIC BLOOD PRESSURE |
| Format/length: | n2 |
| HES item: | |
| National Codes: | |
| Default Codes: |
Notes:
The method used to determine the PERSON OBSERVATION (URINARY ALBUMIN LEVEL).
The urine specimen used to check for albuminuria may be collected in several ways depending on local preference. Staging definitions vary by method so
Derive from the VALUE TYPE recorded for the OBSERVATION TYPE 'Urinary Albumin level'.
The derived values are:
01 - Albumin concentration (µg/L)
02 - Albumin creatinine ratio (µg/mmol)
03 - Timed overnight albumin (µg/min)
04 - 24hr albumin excretion (µg/24hr)
| Context | Alias |
|---|---|
| plural | URINARY ALBUMIN LEVEL TESTING METHODS |
SCDD1 SUMMARY CORE DATA SET FOR DIABETES 
| Summary Core Data Set fpr Diabetes |
SUMMARY CORE DATA SET FOR DIABETES
Demographics
The demographics section contains demographic items necessary to ensure accurate and trouble free liaison between the care provider and the user. All demographic data items will conform to existing national standards.
Diagnosis
The section contains information relating to the type of diabetes and the date of diagnosis. This information has implications for care and management.
Patient Review Data
These data items act as a record of the regular, routine preventative care reviews that form the core of the 'continuing care' component of the patient journey, together with a record of the high level obseravtions and management decisions that are made at any consultation.
Patient Medical History
This section contains those health issues and procedures that may have an effect on, or be a consequence of, the diabetic condition and which will be needed by the patient/clinician in order to manage and monitor care safely and effectively. It refers to chronic concurrent conditions, and also to acute complications associated more directly with the diabetic condition.
| Summary Core Data Set for Diabetes |
The Summary Core Dataset for Diabetes is developed to ensure people with diabetes have up to date records of their risk factors, current management, treatment target achievements and arrangements and outcomes of regular surveillance for complications, to help them monitor their care and make informed choices about their management.
The Summary Core Dataset for Diabetes will also ensure that when people with diabetes meet health care professionals the consultation is fully informed by comprehensive, up to date and accurate information.