| Change Request |
| Reference: | Change Request 316 |
| Version No: | 1.3 |
| Subject: | DSCN 10/2003 - Review of KC61 - Pathology Lab's, etc |
| Type of Change: | Revision of NHS Data Standards |
| Effective Date: | 1 April 2003 |
| Reason for Change: | To update the NHS Data Dictionary Version 2 with the latest revisions to KC61 |
Changes to KC61 will provide more information about laboratories and improve the reporting of cancers diagnosed as a result of screening. Part C2 is not mandatory until 1 April 2004.
Summary of changes:| Class Definitions | |
| PATHOLOGY LABORATORY INVESTIGATION | Change to attributes |
| Attribute Definitions | |
| BIOPSY REFERRAL OUTCOME | Change to description |
| PRIMARY SCREENING | New Attribute |
| Central Return Forms | |
| KC61 1 | Change guidance text |
| KC61 3 | Change guidance text |
| KC61 5 | Change guidance text |
| KC61 6 | New Form |
| Name: | Kevin Shine |
| Date: | 11 March 2003 |
| Sponsor: | Data Standards Team |
Attributes of this Class are:
| O | MARKER LYMPH NODE RESULT | |
| O | PATHOLOGY INVESTIGATION PRIORITY | |
| O | PATHOLOGY RESULT DUE DATE | |
| O | PATHOLOGY RESULT DUE TIME | |
| PRIMARY SCREENING |
A code used to reference an outcome of a REFERRAL FOR BIOPSY. For cervical histology, biopsies are taken after a colposcopy has been performed.
For cervical histology for KC61 purposes, the classification is:
| a. | Cervical Cancer - stage 1B or worse | |
| | ||
| b. | Cervical Cancer - stage 1A | |
| | ||
| c. | Adenocarcinoma in situ/CGIN | |
| | ||
| d. | CIN3 | |
| | ||
| e. | CIN2 | |
| | ||
| f. | CIN1 | |
| | ||
| g. | HPV only | |
| | ||
| h. | No CIN/No HPV | |
| | ||
| i. | Seen in Colposcopy - NAD no biopsy taken | |
| | ||
| j. | Outcome known - none of the above | |
| | ||
| k. | Seen in Colposcopy - result not known | |
| l. | No outcome available | |
| m. | Inadequate biopsy | |
| n. | Non cervical cancers detected | |
For cervical histology for KC65 purposes, the classification is:
| a. | Cancer (including micro-invasive) | |
| b. | Adenocarcinoma in situ | |
| c. | CIN3 | |
| d. | CIN2 | |
| e. | CIN1 | |
| f. | HPV/cervicitus only | |
| g. | No CIN/No HPV (normal) | |
| h. | Inadequate/unsatisfactory biopsy | |
| i. | Result not known by clinic | |
For breast cancer screening for KC62 purposes,the classification is:
| a. | Benign or negative | ||
| b. | Inconclusive | ||
| c. | Positive; i.e. cancer detected | ||
| i. | non-invasice or possibly micro-invasive | ||
| a. | low (DCIS only detected) | ||
| b. | intermediate (DCIS only detected) | ||
| c. | high (DCIS only detected) | ||
| d. | grade not known (DCIS only detected) | ||
| ii. | definitely micro-invasive | ||
| a. | low (DCIS only detected) | ||
| b. | intermediate (DCIS only detected) | ||
| c. | high (DCIS only detected) | ||
| d. | grade not known (DCIS only detected) | ||
| iii. | invasive size not known | ||
| a. | Grade I | ||
| b. | Grade II | ||
| c. | Grade III | ||
| d. | grade not known | ||
| iv. | invasive size known | ||
| a. | Grade I | ||
| b. | Grade II | ||
| c. | Grade III | ||
| d. | grade not known | ||
| v. | invasive status not known | d. | Biopsy not done or result not yet know |
References:
KC65 - Colposcopy Clinics, Referrals, Treatments and Outcomes
KC61: Pathology Laboratories - Cervical Cytology and Outcome of Gynaecological Referrals
KC62: Adult Screening Programmes - Breast Screening
| Context | Alias |
|---|---|
| plural | BIOPSY REFERRAL OUTCOMES |
A classification of whether the PATHOLOGY LABORATORY INVESTIGATION is for primary screening or other reason ('other' may include rapid review, checking, quality assurance and abnormal or clinical reporting).
Primary screening is the first examination of a sample sent for screening.
Classification
| a. | Primary screening | b. | Other reason |
References:
DSCN 03/2003 Cervical Screening Statistics - Revision of Central Statistical Returns: KC61
| Context | Alias |
|---|---|
| plural | PRIMARY SCREENINGS |
| Central Return Form Guidance |
The Department, NHS Cervical Screening Programme (NHSCSP), Regional Offices and trusts require information from PATHOLOGY LABORATORIES on cervical cytology and outcome of referrals.
The Department, NHS Cervical Screening Programme (NHSCSP), Strategic Health Authorities and trusts require information from PATHOLOGY LABORATORIES on cervical cytology and outcome of referrals.
The information helps to monitor the process of achieving the Government's target to reduce the incidence of invasive cervical cancer and to ensure that the screening programme is managed effectively. The information is used to ensure that the laboratory is achieving acceptable standards in examining smears in line with guidance provided by the NHS Cervical Screening Programme.
Information on the return is also used in Public Expenditure Survey (PES) negotiations, resource allocation to the NHS and Departmental accountability.
Information based on the KC61 return is published annually by the Department in the Statistical Bulletin `Cervical Screening Programme'.
KC61 returns are required by all PATHOLOGY LABORATORIEScarrying out cervical cytology within NHS HEALTH CARE PROVIDERS. This applies to independently managed NHS laboratories, including cytopathology laboratories and also private laboratories if they are commissioned to report on smears for the NHS. Each return requires the ORGANISATION CODE and ORGANISATION NAME of the NHS Trust and must be signed by a CONSULTANT in one of the Pathology SPECIALTY. It also requires the PATHOLOGY LABORATORY NAME and PATHOLOGY LABORATORY CODE. Note that PATHOLOGY LABORATORY CODES are maintained and issued by the Organisation Codes Service on behalf of the NHS Cervical Screening Programme.
KC61 returns are required by all
Each return requires the ORGANISATION CODE and ORGANISATION NAME of the NHS Trust and must be signed by a CONSULTANT in one of the Pathology SPECIALTIES. It also requires the PATHOLOGY LABORATORY NAME and PATHOLOGY LABORATORY CODE. Note that
A PATHOLOGY LABORATORY's KC61 return should include only those REQUESTS FOR PATHOLOGY INVESTIGATION received by that laboratory. A REQUEST FOR PATHOLOGY INVESTIGATIONforwarded to another laboratory for primary screening should only be included in the second laboratory's return.
A
Smears re-screened as part of internal or external quality control or for any other reason should not be included in the KC61 return.
Smears re-screened within the same Laboratory as part of internal or external quality control or for any other reason should not be included in the KC61 return. The number of requests sent to or received from another Laboratory for primary screening or other reason should be recorded in Part A3.
Where more than one slide is associated with one
The return KC61 is completed annually and submitted within two months of the end of the period.
Parts A and B of the return relate to all smears reported by the laboratory where the smear was received and registered between 1 April of one year and 31 March of the following year. If this date is not recorded, the CERVICAL SMEAR EXAMINED DATE can be used as a proxy. Part C of the return relates to smears where the date of the smear which led to a referral fell in the first three months of the financial year (April, May and June.)
Parts A and B of the return relate to all smears reported by the laboratory where the smear was received and registered between 1 April of one year and 31 March of the following year. If this date is not recorded, the CERVICAL SMEAR EXAMINED DATE can be used as a proxy. Part C1 of the return relates to smears where the date of the smear which led to a referral fell in the first three months of the financial year (April, May and June). Part C2 is a duplicate of Part C1, but will collect data relating to gynaecological referrals from smears registered during the whole of the financial year prior to the current year.
| Central Return Form Guidance |
Part A2 collects information about the backlog of smears in laboratories.
Part A2 collects information about the backlog of smears in laboratories. The laboratory which receives the original request should issue the report and include the information within this return.
This is the total number of PATHOLOGY LABORATORY INVESTIGATIONS received and registered in:
| Quarter 1 - As at 30 June yyyy (Line 0001) |
| Quarter 2 - As at 30 September yyyy (Line 0002) |
| Quarter 3 - As at 31 December yyyy (Line 0003) |
| Quarter 4 - As at 31 March yyyy (Line 0004) |
The number of results reported are subdivided into the following time periods:
| 0-2 weeks 0-14 days (column 3) |
| 3-4 weeks 15-28 days (column 4) |
| 5-6 weeks 29-42 days (column 5) |
| 7-8 weeks 43-56 days (column 6) |
| 9-10 weeks 57-70 days (column 7) |
| More than 10 weeks over 70 days (column 8) |
The interval to be reported is from the date of receipt of the smear at the laboratory, the SAMPLE RECEIPT DATE, and the date of authorisation of the final report, the PATHOLOGY RESULT REPORTED DATE (for the SAMPLE COLLECTED).
This is the total for all time periods counted in lines 0001 to 0004.
Part A3 records information about the numbers of imported and exported smears dealt with by laboratories.
Part A3 records information about which laboratories import and export smears.
This requires the number of REQUESTS FOR PATHOLOGY INVESTIGATION where the REQUEST FOR DIAGNOSTIC TEST for the primary screening is to be sent to and carried out by another PATHOLOGY LABORATORY.
This requires the number of REQUESTS FOR PATHOLOGY INVESTIGATION where the REQUEST FOR DIAGNOSTIC TEST for the screening is to be sent to and carried out by another PATHOLOGY LABORATORY, sub-divided by details of Laboratory sent to and whether for primary screening or 'other'. 'Other' may include rapid review, checking, abnormal or clinical reporting etc.
This requires the number of REQUEST FOR PATHOLOGY INVESTIGATIONS where the REQUEST FOR DIAGNOSTIC TESTfor the primary screening of the received smear has been sent from another PATHOLOGY LABORATORY.
This requires the number of
Part A3 also requires the number of instances where a single report is derived from more than one sample.
This requires the number of REQUESTS FOR PATHOLOGY INVESTIGATIONS where there is more than one SAMPLE COLLECTED.
This requires the number of
| Central Return Form Guidance |
Part C requires the analysis of the number of women subsequently referred for gynaecological investigation following a smear. This is where the CYTOLOGY SCREENING ACTION TYPE of a SCREENING TEST has a classification of Refer for medical assessment or under medical treatment (Suspend) (S). The date of the smear must be between 1 April and 30 June. The CYTOLOGY RESULT TYPES for each woman is used to allocate her to one of appropriate subdivisions of Most significant result in columns 3 7.
Part C1 requires the analysis of the number of women subsequently referred for gynaecological investigation following a smear. This is where the CYTOLOGY SCREENING ACTION TYPE of a SCREENING TEST has a classification of Refer for medical assessment or under medical treatment (Suspend) (S). The date of the smear must be between 1 April and 30 June of the current data year. The CYTOLOGY RESULT TYPES for each woman is used to allocate her to one of appropriate subdivisions of Most significant result in columns 3 to 9.
Note that severe dyskaryosis in column 7 combines CYTOLOGY RESULT TYPEclassifications of Severe dyskaryosis (cat. 4), Severe dyskaryosis/invasive carcinom a (Cat. 5) and Glandular neoplasia (Cat. 6).
Note that
CYTOLOGY RESULT TYPES with a classification of Negative (cat. 2) are not counted.
The number of Most significant results in the CYTOLOGY RESULT TYPEcolumns (columns 3 - 7) are further analysed by the BIOPSY REFERRAL OUTCOME (lines 0001-0011). For cervical histology, biopsies are taken at colposcopy.
The number of Most significant results in the
Part C also includes the formula to calculate the Positive Predictive Value (PPV) of smears reported as moderate dyskaryosis or worse to enable the laboratory to assess whether or not they are reaching an achievable standard.
Note that Cervical cancer is sub-divided into 'stage 1B or worse' (line 0001) and 'stage 1A' (line 0002) and that there are four options to describe results which are not applicable or not known: Seen in Colposcopy - NAD no biopsy taken' (line 0009), 'Outcone known - none of the above' (line 0010), 'Seen in Colposcopy - result not known' (line 0011) and 'No outcome available' (line 0012).
Part C1 also includes the formula to calculate the Positive Predictive Value (PPV) of smears reported as moderate dyskaryosis or worse to enable the laboratory to assess whether or not they are reaching an achievable standard.
Part C1 includes the formula to calculate Lost to follow-up of smears reported as 'Seen in colposcopy - result not known' (line 0011) and 'No outcome available' (line 0012), as a percentage of the Total.
Provision has been made to record details of non-cervical cancers at the bottom of Part C1.

| Central Return Form Guidance |
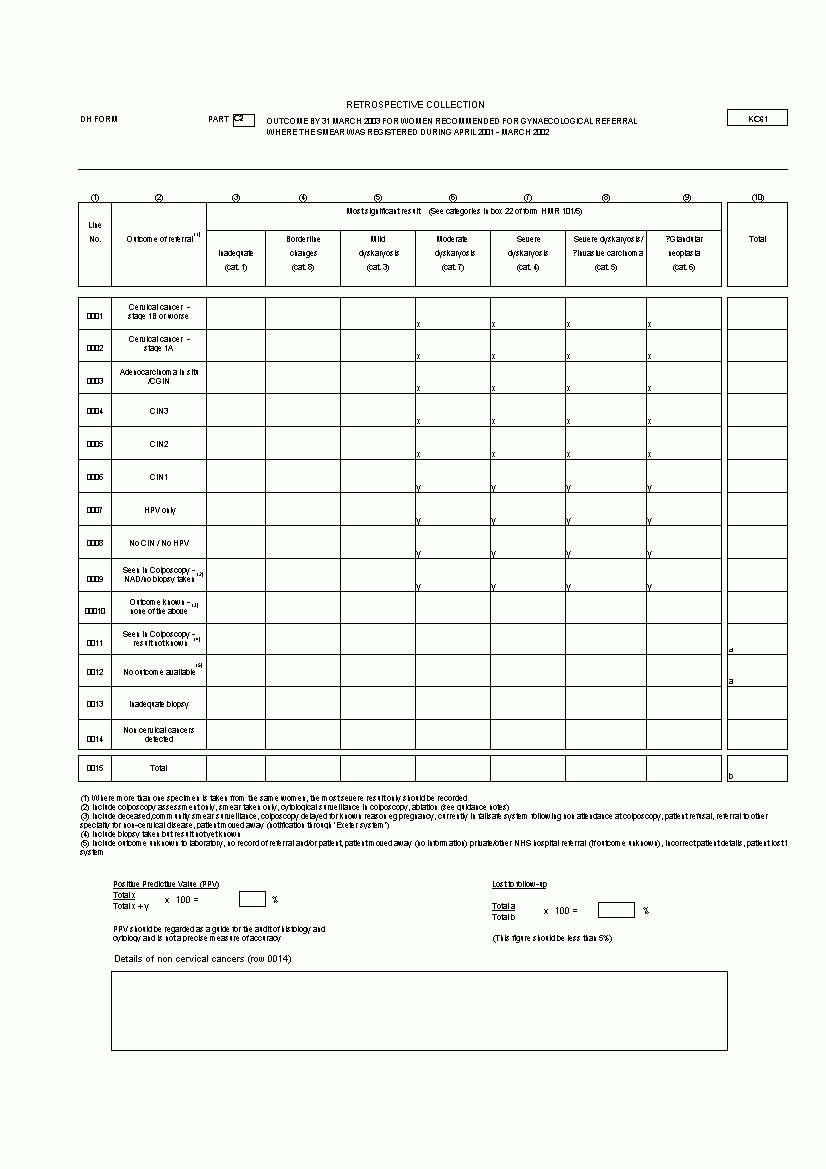
Outcome by 31 March yyyy for Women Recommended for Gynaecological Referral where the Smear was Registered during April yyyy - June yyyy.
Part C2 is a duplicate of Part C1 but will collect data relating to gynaecological referrals from smears registered during the whole of the financial year prior to the current year. This is where the CYTOLOGY SCREENING ACTION TYPE of a SCREENING TEST has a classification of Refer for medical assessment or under medical treatment (Suspend) (S). The date of the smear must be between 1 April and 31 March of the previous data year. The CYTOLOGY RESULT TYPES for each woman is used to allocate her to one of appropriate subdivisions of Most significant result in columns 3 to 9.
Note that
The number of Most significant results in the
Note that Cervical cancer is sub-divided into 'stage 1B or worse' (line 0001) and 'stage 1A' (line 0002) and that there are four options to describe results which are not applicable or not known: Seen in Colposcopy - NAD no biopsy taken' (line 0009), 'Outcone known - none of the above' (line 0010), 'Seen in Colposcopy - result not known' (line 0011) and 'No outcome available' (line 0012).
Part C2 also includes the formula to calculate the Positive Predictive Value (PPV) of smears reported as moderate dyskaryosis or worse to enable the laboratory to assess whether or not they are reaching an achievable standard.
Part C2 includes the formula to calculate Lost to follow-up of smears reported as 'Seen in colposcopy - result not known' (line 0011) and 'No outcome available' (line 0012), as a percentage of the Total.
Provision has been made to record details of non-cervical cancers at the bottom of Part C2.